Incubator at North Brunswick
NEW JERSEY’S LARGEST BIOTECH INCUBATOR
Labs available now !!
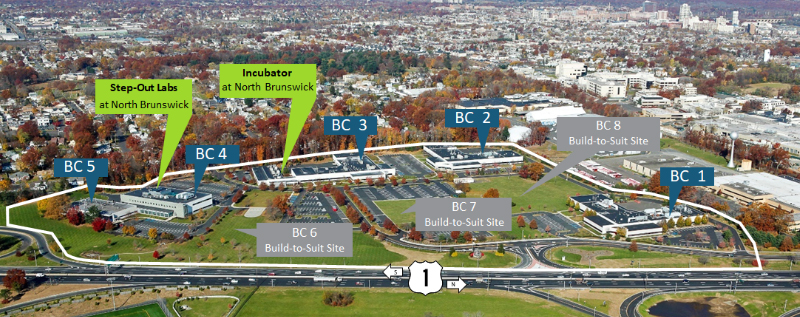
The Incubator at North Brunswick is one of the more significant incubation facilities in the nation dedicated to life sciences and biotechnology companies – a 46,000 sq. ft. biotech wet lab incubator in a 50-acre research park in North Brunswick in the heart of New Jersey’s “Research Corridor.
Strategic Location and Specialized facility

New Jersey is home to the headquarters or major facilities of 14 of the largest biopharmaceutical companies in the world. New Jersey’s life sciences ecosystem employs 115,000 people and includes more than 400 biotechnology companies.
The Incubator at North Brunswick is located in the heart of New Jersey’s “Research Corridor” on the campus of The New Jersey Bioscience Center, a 50-acre research park which offers easy access to both New York City and Philadelphia, as well as close proximity to Rutgers New Jersey Medical School and Princeton University. The New Jersey Bioscience Center itself is home to a number of successful biotech businesses including Chromocell, Allergan, Merial and Orthobond.
The Incubator at North Brunswick is the leading incubator in the region dedicated to life sciences and biotechnology companies. Labs range from approximately 900 – 1,300 sq. ft. and are “plug and play” ready. Tenant companies have shared access to conference rooms, reception services, two kitchens, loading docks, an NMR, glass washing and autoclave. Separate private offices are also available.
Many successful companies have graduated from the Incubator at North Brunswick, including Advaxis, Amicus Therapeutics (Nasdaq: FOLD), Visikol (acquired by CELLINK), Grace Therapeutics (acquired by Acasti Pharmaceuticals), NJ Biopharma and more.



One of the best advantages of locating in the Incubator at North Brunswick is being based at the heart of the entrepreneurial community. In addition to the natural synergies that develop between the life sciences startup companies, there are also many programs designed to educate and support entrepreneurs. The Incubator at North Brunswick community has access to support services including VC training events and 1:1 office hours led by experienced life sciences professionals. The Incubator at North Brunswick advisory board includes representation from pharma, biotech and academia to coach companies into the future.
Businesses located in the Incubator at North Brunswick also have access to a variety of professional and support services including open office hours by professional firms from the life sciences community. In addition to programming, tenant companies also have access to a broad range of common services including conference rooms, reception services, two kitchens, loading docks, an NMR, dishwashing and autoclave. Separate private offices are available for rent to biotech companies that are not in need of lab space.
NJ Ignite
The NJ Ignite Program can provide approved qualified biotech companies with up to 5 months free rent. To be eligible for NJ Ignite at the Incubator at North Brunswick, the company must meet the following requirements:
- A management team or principal(s) having substantial technical experience. Must have demonstrated progress in advancing the technology towards commercial application. Preference to leadership with past success leading a commercial life sciences company.
- Only companies focused on commercializing innovative in-licensed academic and/or medical center-based innovative discovery technologies will be considered. In addition, companies with IP acquired directly from an academic institution and/or medical center are eligible. Eligible technology categories include: i) new chemical entity (NCE) drug discovery technologies ii) diagnostics iii) devices iv) digital health v)biologics. Preference to in-licensed new chemical entity (NCE) drug discovery technologies. Eligibility extends to technologies in-licensed from in or out of state academic institutions or academic medical centers. The following are not considered: R&D services, 505b2, generics.
- Companies must have a funding commitment to sustain them through, at least, one year of projected operations. . May include funding from foundations, angels and government sources (SBIRs) and/or funding from a consortium of life sciences VC firms having track records of success.
- Corporate ownership structure may include joint ventures and strategic partnerships and strategic alliances. The entity applying must have the opportunity to derive financial benefit from the in-licensed or owned technology. If the applicant is not a direct owner of the technology, applicant must have over a 25% ownership stake in the company that owns or has in-licensed the technology.
- A management team or principal(s) having substantial technical experience. Must have demonstrated progress in advancing the technology towards commercial application. Preference to leadership with past success leading a commercial life sciences company.
- Only companies focused on commercializing innovative in-licensed academic and/or medical center-based innovative discovery technologies will be considered. In addition, companies with IP acquired directly from an academic institution and/or medical center are eligible. Eligible technology categories include: i) new chemical entity (NCE) drug discovery technologies ii) diagnostics iii) devices iv) digital health v)biologics. Preference to in-licensed new chemical entity (NCE) drug discovery technologies. Eligibility extends to technologies in-licensed from in or out of state academic institutions or academic medical centers. The following are not considered: R&D services, 505b2, generics.
- Companies must have a funding commitment to sustain them through, at least, one year of projected operations. . May include funding from foundations, angels and government sources (SBIRs) and/or funding from a consortium of life sciences VC firms having track records of success.
- Corporate ownership structure may include joint ventures and strategic partnerships and strategic alliances. The entity applying must have the opportunity to derive financial benefit from the in-licensed or owned technology. If the applicant is not a direct owner of the technology, applicant must have over a 25% ownership stake in the company that owns or has in-licensed the technology.
Newsletter- BCI Connection
Graduates of the Incubator at North Brunswick in the news

additional Information
Contact us

lharcum@njeda.com

rszeliga@njeda.com

ccostello@njeda.com